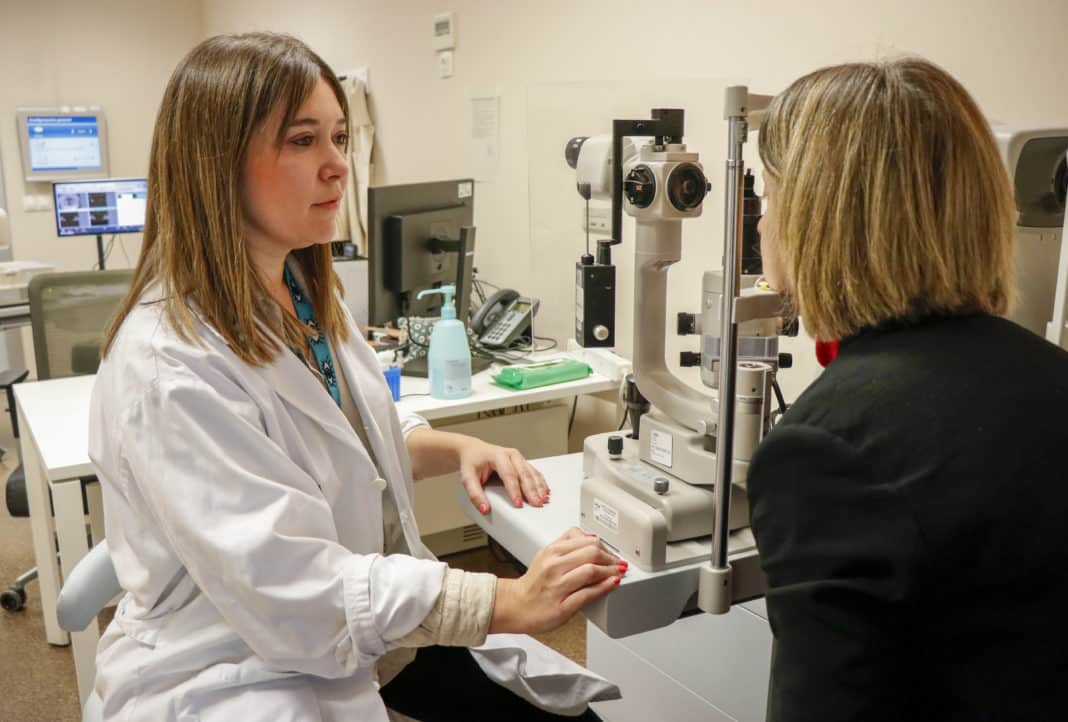
The Ophthalmology services of several hospitals in the Valencian Community have incorporated a new treatment, topical monotherapy with polyhexamide 0.08%, against a parasitic eye infection related to the misuse and poor hygiene of contact lenses, and which can lead to compromised vision.
This pathology is not common, but if not treated in time, it can cause visual impairment and even blindness.
The antimicrobial agent, polyhexamide, has already been successfully applied at a concentration of 0.08% to a total of 17 patients in the Valencian Community. Specifically, the La Fe Hospital has treated eight patients, the Clinical Hospital four, the Gandia Hospital one patient, Doctor Balmis has treated three people and the Sant Joan Hospital has used the drug for a complex case that affected to both eyes.
Acanthamoeba is a free-living amoeba present in nature, which in some people can cause damage through keratitis and, in the worst cases, blindness.
The incidence of Acanthamoeba keratitis has increased in recent years due to the increase in the number of people wearing contact lenses, their inadequate disinfection, as well as outdoor activities and the use of contact lenses in spas or swimming pools. The symptoms of amoebic keratitis can be eye pain, red eyes, blurred vision or loss of vision, as detailed by Ana Hervás, an ophthalmologist at Hospital La Fe.
As explained by the specialist, “the measures that must be adopted to prevent this infection consist of using contact lenses properly and disinfecting them correctly, as well as avoiding their use in swimming pools or spas.”
The head of Ophthalmology at La Fe, Enrique España, said that, in the event of the slightest suspicion, go to the specialist for an early diagnosis using cultures and PCR techniques, and treatment.”
Until the arrival in Europe in 2023 of polyhexamide 0.08% in the form of single-dose eye drops, hospital pharmacies with the capacity to do so combined this active ingredient with others to develop master formulas and treat this eye infection, one of the most serious and painful.
After clinical trials that prove its safety and effectiveness, the Hospital Pharmacy services have now acquired the eye drops ready for administration through the Spanish Medicines Agency.