- President Putin says it is “effective” and generates “stable immunity”
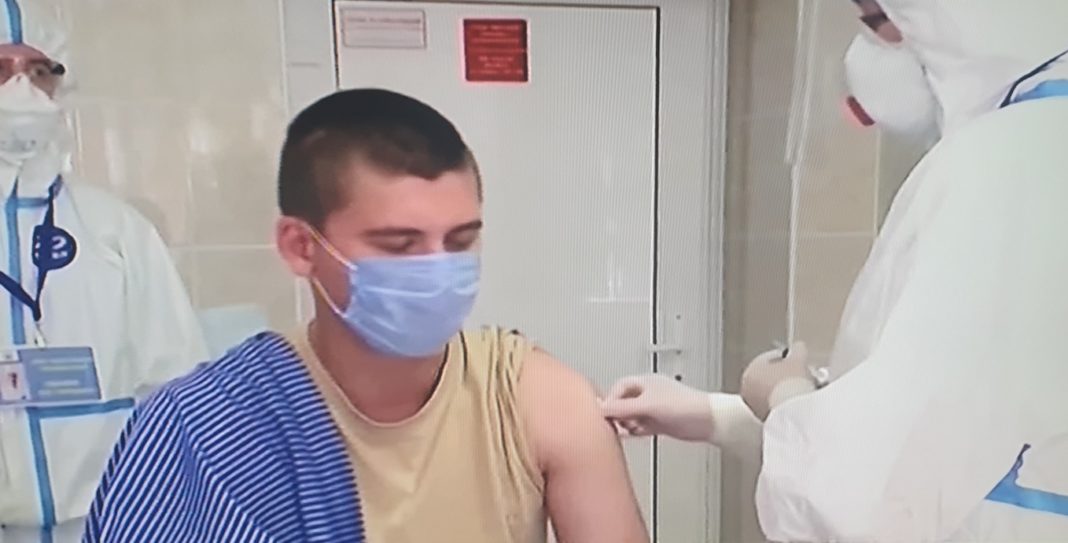
Russian President Vladimir Putin announced on Tuesday that his daughter has been vaccinated against the coronavirus with a new Russian vaccine, which has already been registered and approved for use. He added that the vaccine will soon be mass-produced and administered to the general public.
Putin said in a meeting with his Cabinet of Ministers, “This morning a vaccine against the new coronavirus was registered for the first time in the world.”
The announcement made by Putin coincides with the deadlines that he has announced since recent weeks, where it was said that the vaccine would be ready for use in August. According to the president, the Russian vaccine is “effective”, it has passed all the necessary tests and it achieves a “stable immunity” against COVID-19.
Putin also said that his own daughter had received the vaccine and he is confident that mass production will start soon so that it can soon be available to all Russians. Last week, the Kremlin pointed to October as a possible start date for a national coronavirus vaccination campaign.
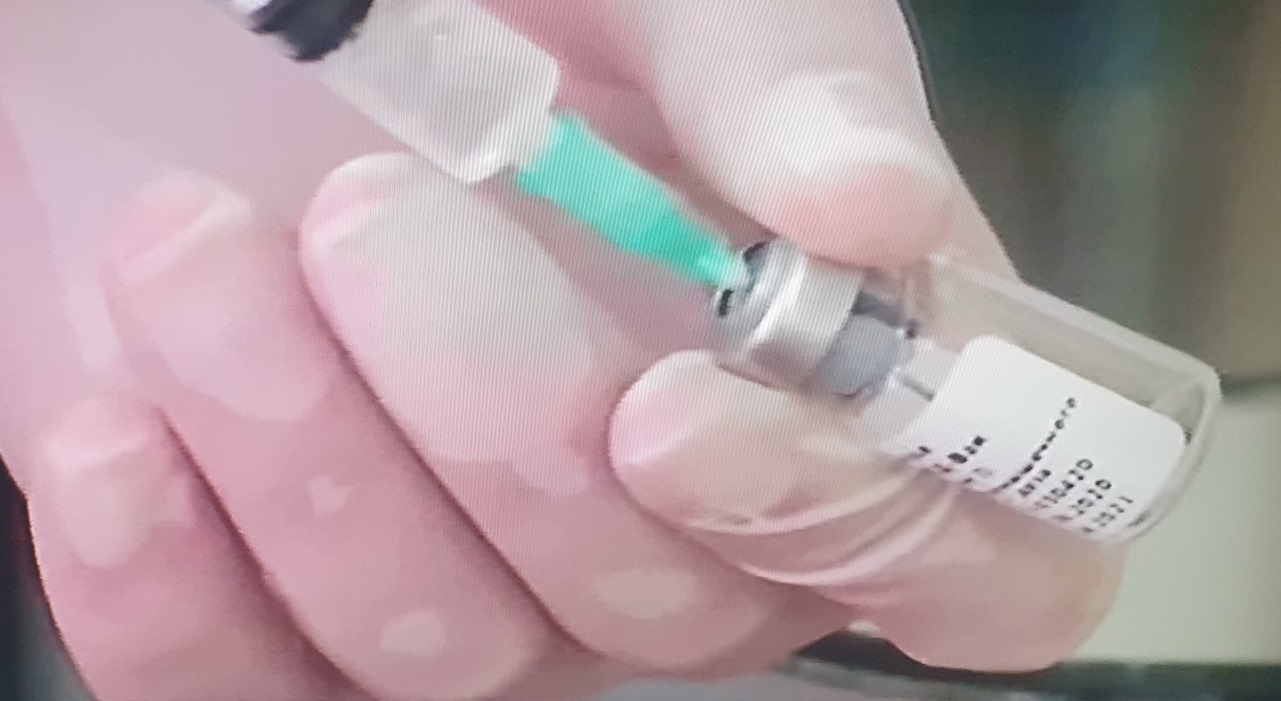
Hours earlier, the head of Epidemiology at the Russian Ministry of Health, Nikolai Briko, had said that “there are no reasons” to postpone the registration of the coronavirus vaccine developed by the NF Gamaleya research centre.
“Perhaps the process has been accelerated by the fact that the vaccine has not been developed by chance,” said Briko, claiming that it is the result of “serious” work.
However, the Russian dash for a vaccine has already raised international concerns that Moscow is cutting corners on testing to score political and propaganda points.
Vaccines are required to go through three stages of human testing before being approved for widespread use. The first two phases test the vaccine on relatively small groups of people to see if it causes harm and if it stimulates the immune system. The last phase, known as Phase 3, compares the vaccine to a placebo in thousands of people.
Experts say that this final phase has not yet been carried out. It is the only way to know with statistical certainty whether a vaccine prevents an infection. And because it’s testing a much larger group of people, a Phase 3 trial can also pick up more subtle side effects of a vaccine that earlier trials could not.
Dr Michael Head Senior Research Fellow in Global Health at Southampton University said that the rush to start using the vaccine before Phase 3 trials – which usually last for months and involve thousands of people – could backfire.

Russia has diagnosed less than 5,000 new cases of coronavirus in the last day, the first time that this figure has fallen below that level since the end of April.
“In the last 24 hours in Russia, 4,945 cases of COVID-19 were confirmed in 84 regions,” the Russian Epidemiology centre has said. To find a similar figure, we must go back to April 23, when 4,774 new positives were diagnosed.
Russia is the fourth most affected country in the world by the pandemic, only behind the United States, Brazil and India.